

Photograph © Dr Jon Russ See http://www.nathusius.org.uk/ for further details of Nathusius' pipistrelles. |
||
|
The diagram below gives important average body measurements for Nathusius' pipistrelle (Greenaway & Hutson, 1990). |
||
|
||
|
||
|
 |
|
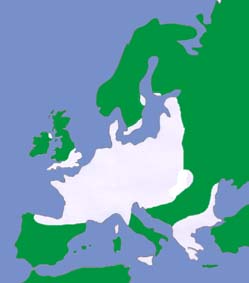
| The British and European distributions are shown by the white areas of the maps above (as given by Richardson, 2000 and Mitchell-Jones et al., 1999). | ||
|
||
|
||
|
||
|
||
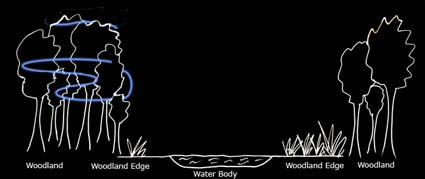
| The diet of Nathusius' pipistrelles mainly consists of aquatic Diptera, particularly Chironomidae (Vaughan, 1997). | ||
 |
||
|
Marked in blue on the diagram above is a typical foraging path of Nathusius' pipistrelles (based on Russ, 1999). |
||
|
||
|
|
||
|
To listen to the call of the Nathusius' pipistrelle click here Size of sound file: 74.6 KB Sound file provided by Jon Russ. |
||
| For details of how the echolocation calls were recorded click here. | ||
|
Average values for a Nathusius' pipistrelle echolocation call, as given by Vaughan et al. (1997), are listed below: Interpulse interval: 103.0 ms Call duration: 7.7 ms Minimum frequency: 39.2 kHz Maximum frequency: 48.5 kHz The spectrogram on the left shows clear frequency modulation, with the call beginning at high frequency and ending at a lower frequency. The end of the call consists of a continuous freqency component. The power spectrum on the left shows that the maximum power of the call is at a frequency of approximately 39 kHz. Mating calls often produced whilst the bats are inactive on trees or buildings and whilst flying. Kalko (1995) found four stages to the foraging behaviour of pipistrelles: search flight, approach flight, capture and retrieval of prey. Changes in the echolocation call were correlated with these changes in flight behaviour. There is a shallow-modulated component in the echolocation call during search phase which may enable better detection of prey. This shallow-modulated component is not found in the echolocation call during the approach phase. During the approach phase a steep frequency-modulated call is used to assess the distance to the prey item. In this phase the pulse interval and duration decrease as the target is approached. During the terminal phase steep frequency-modulated calls are used to locate the prey item precisely. Again, the pulse interval and duration decrease as the target is approached. The information contained in the call per unit time is increased through the use of a high call repetition rate. By altering the duration of the echolocation call, pipistrelles are able to avoid overlap between emitted signals and returning echos. Using a call with a duration of 6-10 ms, Kalko (1995) suggests that pipistrelles should be able to detect prey at a minimum distance of 1.12-1.70 m. Kalko & Schnitzler (1993) studied search flight echolocation of Pipistrellus species. During the search phase echolocation type corresponded to habitat type. Where obstacles were greater than 5m away from the bat the call was less than 15 kHz in width. In cluttered habitats and when the bats were turning the call was more than 15 kHz in width. Prey was only detected when there was no overlap between the emitted call and the received echo. Nathusius' pipistrelles emit two types of social calls; song-like calls at the mating roost, during flight and when foraging, and cheep-like calls during flight (Pfalzer & Kusch, 2003).
Click here to listen to the social call of Nathusius' pipistrelle. Size of sound file: 153 KB |
||
|
|
||
|
||
 © School of Biological Sciences, University of Bristol 2005. Last modified 24th February 2005. © School of Biological Sciences, University of Bristol 2005. Last modified 24th February 2005. |
||